Page 716 - TNFlipTest
P. 716
NP16 Nephrology
Acid-Base Disorders Toronto Notes 2019 Acid-Base Disorders
• acid-basehomeostasisinfluencesproteinfunctionandcancriticallyaffecttissueandorganfunction with consequences to cardiovascular, respiratory, metabolic, and CNS function
• seeRespirology,R6formoreinformationonrespiratoryacidosis/alkalosis
• normal concentration of HCO3– = 24 mEq/L (range: 22-30 mEq/L)
• normal pCO2 = 40 mmHg (range: 36-44 mmHg)
• eachacid-basedisorderhasanappropriatecompensation
■ inadequate compensation or overcompensation can indicate the presence of a second acid-base disorder (e.g. in metabolic acidosis, inadequate compensation means there is also respiratory acidosis; overcompensation means there is also respiratory alkalosis)
Low (pH <7.35)
Acidemia
Low High HCO3– pCO2
pH
Normal
No Disturbance or
Mixed Disturbance
Mixed if pCO2 + HCO3– change in opposite directions or plasma AG wide
High HCO3–
High (pH >7.45)
Alkalemia
Respiratory alkalosis
Acute:10 pCO2 =2 HCO3– Chronic:10 pCO2 =5 HCO3–
Low pCO2
Metabolic acidosis
Respiratory acidosis
Metabolic alkalosis
10 HCO3– =5-7 pCO2
Acute:10 pCO2 =1 HCO3– Chronic:10 pCO2 =3 HCO3–
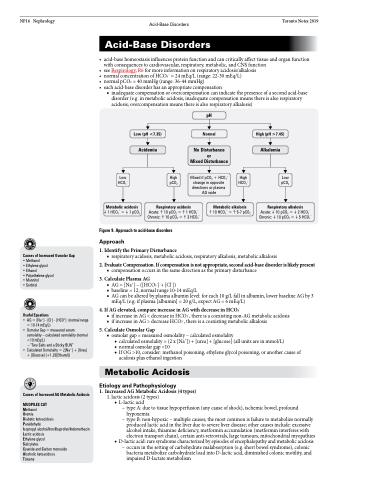
1. Identify the Primary Disturbance
1 HCO3– =1 pCO2
Figure 9. Approach to acid-base disorders
Approach
Causes of Increased Osmolar Gap
• Methanol
• Ethylene glycol
• Ethanol
• Polyethelene glycol • Mannitol
• Sorbitol
Useful Equations
• AG = [Na+] - [Cl–] - [HCO3-] (normal range = 10-14 mEq/L)
• Osmolar Gap = measured serum osmolality – calculated osmolality (normal <10 mEq/L)
– “Two Salts and a Sticky BUN”
• Calculated Osmolality = 2[Na+] + [Urea] + [Glucose] (+1.25[Ethanol])
Causes of Increased AG Metabolic Acidosis
MUDPILES CAT
Methanol
Uremia
Diabetic ketoacidosis
Paraldehyde
Isopropyl alcohol/Iron/Ibuprofen/Indomethacin Lactic acidosis
Ethylene glycol
Salicylates
Cyanide and Carbon monoxide Alcoholic ketoacidosis Toluene
■ respiratory acidosis, metabolic acidosis, respiratory alkalosis, metabolic alkalosis
2. Evaluate Compensation. If compensation is not appropriate, second acid-base disorder is likely present
■ compensation occurs in the same direction as the primary disturbance
3. Calculate Plasma AG
■ AG = [Na+] – ([HCO3-] + [Cl-])
■ baseline = 12, normal range 10-14 mEq/L
■ AG can be altered by plasma albumin level: for each 10 g/L fall in albumin, lower baseline AG by 3
mEq/L (e.g. if plasma [albumin] = 20 g/L, expect AG = 6 mEq/L)
4. If AG elevated, compare increase in AG with decrease in HCO3-
■ if increase in AG < decrease in HCO3-, there is a coexisting non-AG metabolic acidosis ■ if increase in AG > decrease HCO3-, there is a coexisting metabolic alkalosis
5. Calculate Osmolar Gap
■ osmolar gap = measured osmolality – calculated osmolality
◆ calculated osmolality = (2 x [Na+]) + [urea] + [glucose] (all units are in mmol/L)
◆ normal osmolar gap <10
◆ If OG >10, consider: methanol poisoning, ethylene glycol poisoning, or another cause of
acidosis plus ethanol ingestion
Metabolic Acidosis
Etiology and Pathophysiology
1. Increased AG Metabolic Acidosis (4 types)
1. lactic acidosis (2 types) ◆ L-lactic acid
– type A: due to tissue hypoperfusion (any cause of shock), ischemic bowel, profound hypoxemia
– type B: non-hypoxic – multiple causes; the most common is failure to metabolize normally produced lactic acid in the liver due to severe liver disease; other causes include: excessive alcohol intake, thiamine deficiency, metformin accumulation (metformin interferes with electron transport chain), certain anti-retrovirals, large tumours, mitochondrial myopathies
◆ D-lactic acid: rare syndrome characterized by episodes of encephalopathy and metabolic acidosis – occurs in the setting of carbohydrate malabsorption (e.g. short bowel syndrome), colonic
bacteria metabolize carbohydrate load into D-lactic acid, diminished colonic motility, and impaired D-lactate metabolism