Page 134 - TNFlipTest
P. 134
CP12 Clinical Pharmacology
Common Drug Endings
Toronto Notes 2019
Common Drug Endings
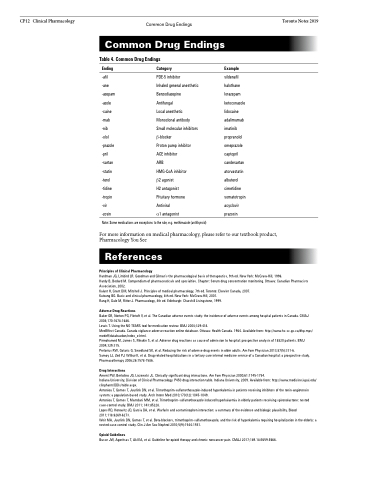
Table 4. Common Drug Endings
Ending Category
-afil PDE-5 inhibitor
-ane Inhaled general anesthetic -azepam Benzodiazepine
-azole Antifungal
-caine Local anesthetic
-mab Monoclonal antibody
-nib Small molecular inhibitors -olol β-blocker
-prazole Proton pump inhibitor
-pril ACE inhibitor
-sartan ARB
-statin HMG-CoA inhibitor
-terol β2 agonist
-tidine H2 antagonist
-tropin Pituitary hormone
-vir Antiviral
-zosin α1 antagonist
Note: Some medications are exceptions to the rule, e.g. methimazole (antithyroid)
Example
sildenafil halothane lorazepam ketoconazole lidocaine adalimumab imatinib propranolol omeprazole captopril candesartan atorvastatin albuterol cimetidine somatotropin acyclovir prazosin
For more information on medical pharmacology, please refer to our textbook product, Pharmacology You See
References
Principles of Clinical Pharmacology
Hardman JG, Limbird LR. Goodman and Gilman’s the pharmacological basis of therapeutics, 9th ed. New York: McGraw-Hill, 1996.
Hardy B, Bedard M. Compendium of pharmaceuticals and specialties. Chapter: Serum drug concentration monitoring. Ottawa: Canadian Pharmacists Association, 2002.
Kalant H, Grant DM, Mitchell J. Principles of medical pharmacology, 7th ed. Toronto: Elsevier Canada, 2007.
Katzung BG. Basic and clinical pharmacology, 8th ed. New York: McGraw-Hill, 2001.
Rang H, Dale M, Ritter J. Pharmacology, 4th ed. Edinburgh: Churchill Livingstone, 1999.
Adverse Drug Reactions
Baker GR, Norton PG, Flintoft V, et al. The Canadian adverse events study: the incidence of adverse events among hospital patients in Canada. CMAJ 2004;170:1678-1686.
Lewis T. Using the NO TEARS tool for medication review. BMJ 2004;329:434.
MedEffect Canada. Canada vigilance adverse reaction online database. Ottawa: Health Canada. 1964. Available from: http://www.hc-sc.gc.ca/dhp-mps/ medeff/databasdon/index_e.html.
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ 2004;329:315.
Pretorius RW, Gataric G, Swedlund SK, et al. Reducing the risk of adverse drug events in older adults. Am Fam Physician 2013;87(5):331-6.
Samoy LJ, Zed PJ, Wilbur K, et al. Drug-related hospitalizations in a tertiary care internal medicine service of a Canadian hospital: a prospective study. Pharmacotherapy 2006;26:1578-1586.
Drug Interactions
Ament PW, Bertolino JG, Liszewski JL. Clinically significant drug interactions. Am Fam Physician 2000;61:1745-1754.
Indiana University, Division of Clinical Pharmacology. P450 drug interaction table. Indiana University, 2009. Available from: http://www.medicine.iupui.edu/ clinpharm/DDIs/table.aspx.
Antoniou T, Gomes T, Juurlink DN, et al. Trimethoprim-sulfamethoxazole-induced hyperkalemia in patients receiving inhibitors of the renin-angiotensin system: a population-based study. Arch Intern Med 2010;170(12):1045-1049.
Antoniou T, Gomes T, Mamdani MM, et al. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. BMJ 2011; 343:d5228.
Lopes RD, Horowitz JD, Garcia DA, et al. Warfarin and acetaminophen interaction: a summary of the evidence and biologic plausibility. Blood 2011;118:6269-6273.
Weir MA, Juurlink DN, Gomes T, et al. Beta-blockers, trimethoprim-sulfamethoxazole, and the risk of hyperkalemia requiring hospitalization in the elderly: a nested case-control study. Clin J Am Soc Nephrol 2010;5(9):1544-1551.
Opioid Guidelines
Busse JW, Agoritsas T, Akl EA, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189.18:E659-E666.